Abstract
Purpose
We report the clinical efficacy of sequential applications of 0.3% and 0.15% unpreserved hyaluronic acid (HA) for the treatment of dry eye disease (DED).
Study design
Randomized clinical trial.
Methods
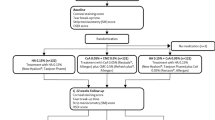
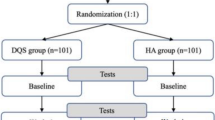
Patients over 19 years of age with DED level 2 or higher, corneal fluorescein staining (CFS) score > 1, and tear break-up time (TBUT) < 10 s were included. Seventy-six patients were randomly assigned to the 0.15% HA group, 0.3% HA group, or combination group. Each group applied two drops of 0.15% or 0.3% HA, or a single drop of both 0.3% and 0.15% HA. Patients were evaluated using the ocular surface disease index (OSDI), CFS and conjunctival fluorescein stain score, TBUT, and blurring/discomfort after application at baseline, 4 weeks, and 8 weeks.
Results
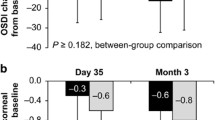
The combination group had the greatest improvement in CFS score from baseline to 8 weeks, compared with the 0.15% and 0.3% HA group (p < 0.001). The combined CFS-OSDI responder rates of the combination group (CFS score = 0 and OSDI ≥ 50% improvement at 8 weeks) were significantly higher than those of the 0.15% and 0.3% groups (p = 0.037). At 4 and 8 weeks, blurring after application in both the 0.3% and combination groups was significantly higher than in the 0.15% group, despite no difference between the 0.3% and combination groups. There were no differences in CFS and conjunctival staining score, TBUT, or OSDI within the three groups at baseline, 4 weeks, and 8 weeks.
Conclusions
Sequential application of 0.3% and 0.15% HA improved symptoms/signs in moderate to severe DED patients.





Similar content being viewed by others
References
Jones L, Downie LE, Korb D, Benitez-Del-Castillo JM, Dana R, Deng SX, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15:575–628.
Nakamura M, Hikida M, Nakano T, Ito S, Hamano T, Kinoshita S. Characterization of water retentive properties of hyaluronan. Cornea. 1993;12:433–6.
Nishida T, Nakamura M, Mishima H, Otori T. Hyaluronan stimulates corneal epithelial migration. Exp Eye Res. 1991;53:753–8.
Inoue M, Katakami C. The effect of hyaluronic acid on corneal epithelial cell proliferation. Invest Ophthalmol Vis Sci. 1993;34:2313–5.
Gomes JA, Amankwah R, Powell-Richards A, Dua HS. Sodium hyaluronate (hyaluronic acid) promotes migration of human corneal epithelial cells in vitro. Br J Ophthalmol. 2004;88:821–5.
Zhong J, Deng Y, Tian B, Wang B, Sun Y, Huang H, et al. Hyaluronate acid-dependent protection and enhanced corneal wound healing against oxidative damage in corneal epithelial cells. J Ophthalmol. 2016;2016:6538051.
Labetoulle M, Chiambaretta F, Shirlaw A, Leaback R, Baudouin C. Osmoprotectants, carboxymethylcellulose and hyaluronic acid multi-ingredient eye drop: a randomised controlled trial in moderate to severe dry eye. Eye (Lond). 2017;31:1409–16.
She Y, Li J, Xiao B, Lu H, Liu H, Simmons PA, et al. Evaluation of a novel artificial tear in the prevention and treatment of dry eye in an animal model. J Ocul Pharmacol Ther. 2015;31:525–30.
Lee JH, Ahn HS, Kim EK, Kim TI. Efficacy of sodium hyaluronate and carboxymethylcellulose in treating mild to moderate dry eye disease. Cornea. 2011;30:175–9.
Moon SW, Hwang JH, Chung SH, Nam KH. The impact of artificial tears containing hydroxypropyl guar on mucous layer. Cornea. 2010;29:1430–5.
Rangarajan R, Kraybill B, Ogundele A, Ketelson HA. Effects of a hyaluronic acid/hydroxypropyl guar artificial tear solution on protection, recovery, and lubricity in models of corneal epithelium. J Ocul Pharmacol Ther. 2015;31:491–7.
Labetoulle M, Schmickler S, Galarreta D, Bohringer D, Ogundele A, Guillon M, et al. Efficacy and safety of dual-polymer hydroxypropyl guar- and hyaluronic acid-containing lubricant eyedrops for the management of dry-eye disease: a randomized double-masked clinical study. Clin Ophthalmol. 2018;12:2499–508.
You IC, Li Y, Jin R, Ahn M, Choi W, Yoon KC. Comparison of 0.1%, 0.18%, and 0.3% hyaluronic acid eye drops in the treatment of experimental dry eye. J Ocul Pharmacol Ther. 2018;34:557–64.
Park Y, Song JS, Choi CY, Yoon KC, Lee HK, Kim HS. A randomized multicenter study comparing 0.1%, 0.15%, and 0.3% sodium hyaluronate with 0.05% cyclosporine in the treatment of dry eye. J Ocul Pharmacol Ther. 2017;33:66–72.
Lee HS, Ji YS, Yoon KC. Efficacy of hypotonic 0.18% sodium hyaluronate eye drops in patients with dry eye disease. Cornea. 2014;33:946–51.
Aragona P, Di Stefano G, Ferreri F, Spinella R, Stilo A. Sodium hyaluronate eye drops of different osmolarity for the treatment of dry eye in Sjogren’s syndrome patients. Br J Ophthalmol. 2002;86:879–84.
Papa V, Aragona P, Russo S, Di Bella A, Russo P, Milazzo G. Comparison of hypotonic and isotonic solutions containing sodium hyaluronate on the symptomatic treatment of dry eye patients. Ophthalmologica. 2001;215:124–7.
Zheng X, Goto T, Ohashi Y. Comparison of in vivo efficacy of different ocular lubricants in dry eye animal models. Invest Ophthalmol Vis Sci. 2014;55:3454–60.
Oechsner M, Keipert S. Polyacrylic acid/polyvinylpyrrolidone bipolymeric systems. I. Rheological and mucoadhesive properties of formulations potentially useful for the treatment of dry-eye-syndrome. Eur J Pharm Biopharm. 1999;47:113–8.
Pires NR, Cunha PL, Maciel JS, Angelim AL, Melo VM, de Paula RC, et al. Sulfated chitosan as tear substitute with no antimicrobial activity. Carbohydr Polym. 2013;91:92–9.
The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf.2007;5:75-92
Lemp MA. Report of the National Eye Institute/Industry workshop on clinical trials in dry eyes. CLAO J. 1995;21:221–32.
Wolffsohn JS, Arita R, Chalmers R, Djalilian A, Dogru M, Dumbleton K, et al. TFOS DEWS II diagnostic methodology report. Ocul Surf. 2017;15:539–74.
Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the ocular surface disease index. Arch Ophthalmol. 2000;118:615–21.
Stapleton F, Alves M, Bunya VY, Jalbert I, Lekhanont K, Malet F, et al. TFOS DEWS II epidemiology report. Ocul Surf. 2017;15:334–65.
Sullivan DA, Rocha EM, Aragona P, Clayton JA, Ding J, Golebiowski B, et al. TFOS DEWS II sex, gender, and hormones report. Ocul Surf. 2017;15:284–333.
Salzillo R, Schiraldi C, Corsuto L, D’Agostino A, Filosa R, De Rosa M, et al. Optimization of hyaluronan-based eye drop formulations. Carbohydr Polym. 2016;153:275–83.
Zheng X, Goto T, Shiraishi A, Ohashi Y. In vitro efficacy of ocular surface lubricants against dehydration. Cornea. 2013;32:1260–4.
van Setten GB, Stachs O, Dupas B, Turhan SA, Seitz B, Reitsamer H, et al. High molecular weight hyaluronan promotes corneal nerve growth in severe dry eyes. J Clin Med. 2020;9:3799.
Müller-Lierheim WGK. Why chain length of hyaluronan in eye drops matters. Diagnostics (Basel). 2020;10:511.
Kojima T, Nagata T, Kudo H, Müller-Lierheim WGK, van Setten GB, Dogru M, et al. The effects of high molecular weight hyaluronic acid eye drop application in environmental dry eye stress model mice. Int J Mol Sci. 2020;21:3516.
Johnson ME, Murphy PJ, Boulton M. Effectiveness of sodium hyaluronate eyedrops in the treatment of dry eye. Graefes Arch Clin Exp Ophthalmol. 2006;244:109–12.
Shimmura S, Ono M, Shinozaki K, Toda I, Takamura E, Mashima Y, et al. Sodium hyaluronate eyedrops in the treatment of dry eyes. Br J Ophthalmol. 1995;79:1007–11.
Prabhasawat P, Tesavibul N, Kasetsuwan N. Performance profile of sodium hyaluronate in patients with lipid tear deficiency: randomised, double-blind, controlled, exploratory study. Br J Ophthalmol. 2007;91:47–50.
Behrens A, Doyle JJ, Stern L, Chuck RS, McDonnell PJ, Azar DT, et al. Dysfunctional tear syndrome: a Delphi approach to treatment recommendations. Cornea. 2006;25:900–7.
Eliason JA, Maurice DM. Staining of the conjunctiva and conjunctival tear film. Br J Ophthalmol. 1990;74:519–22.
Acknowledgements
This work was equally supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2021R1A2C2013939 and 2021R1C1C1011528).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
J. H. Jun, None; S. P. Bang, None; H. S. Park, None; D. Yoon, None; J. Y. Ahn, None; S. J. Kim, None; H. K. Kim, None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Corresponding Author: Hong Kyun Kim
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Jun, J.H., Bang, S.P., Park, H.S. et al. A randomized multicenter clinical evaluation of sequential application of 0.3% and 0.15% hyaluronic acid for treatment of dry eye. Jpn J Ophthalmol 66, 58–67 (2022). https://doi.org/10.1007/s10384-021-00885-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-021-00885-x